Ferroptosis is a newly discovered form of programmed cell death in recent years, which is mainly marked by the accumulation of iron-dependent lipid peroxidation. It has been confirmed that ferroptosis is related to various metabolic factors such as amino acids, lipids, and peroxides, and multiple metabolic pathways are involved in the regulation of ferroptosis. However, how cells respond to and regulate ferroptotic stress in the early stages of ferroptosis remains to be studied.
On May 3, 2022, the team of Professor Chen Quan and Professor Zhou Jun from the College of Life Sciences and State Key Laboratory of Medical Chemical Biology of Nankai University jointly published a research result entitled Dynamic O-GlcNAcylation coordinates ferritinophagy and mitophagy to activate ferroptosis in Cell Discovery. This study found that O-GlcNAcylation modification of proteins plays an important role in the process of ferroptosis, revealing the regulatory mechanism of cells responding to ferroptosis stress in the early stage of ferroptosis.
The researchers found that O-GlcNAcylation modification, acting as a nutrient and baroreceptor of cells, rapidly increased under the stress of ferroptosis; if inhibited with a small molecule inhibitor or knocked down the enzyme that catalyzes O-GlcNAcylation modification with siRNA, the modification was prevented from increasing. Will greatly promote the process of iron death. Further studies found that inhibition of O-GlcNAcylation can promote iron autophagy and release iron stored in ferritin; a large amount of released iron is transported to mitochondria as a buffer, however, after reducing O-GlcNAcylation, mitochondria are fragmented. , and further mitophagy occurs, releasing iron from mitochondria to promote the process of ferroptosis.
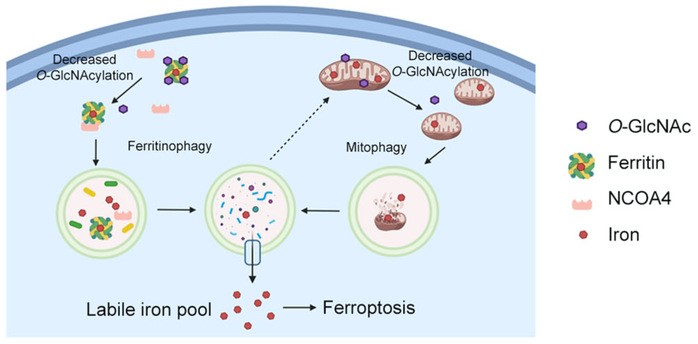
In further studies, the researchers found that the protein ferritin FTH, which stores iron in cells, has an O-GlcNAcylation modification, and the modification site is at S179 of FTH. When S179 is mutated and cannot be modified by O-GlcNAcylation, FTH-S179A greatly increases the binding to its autophagy receptor NCOA4, is transported to lysosomes for degradation, and releases the iron stored therein, thereby enhancing the cellular response to iron. Sensitivity to death.
In conclusion, this study focused on the measures taken by cells to cope with ferroptosis under ferroptotic stress, revealed the coping mechanism of cells in the early stage of ferroptosis, and expanded the regulatory network of ferroptosis. This study also has certain guiding significance for the further response to ferroptosis-related diseases.
Yu Fan, a postdoctoral fellow at the School of Life Sciences of Nankai University, and Zhang Qianping, a doctoral student, are the co-first authors of this study, and Professor Chen Qan and Professor Zhou Jun are co-corresponding authors.
Paper link: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9065108/