On May 26, Professor Gong Hongri and academician Rao Zihe from Nankai University published a research paper entitled “Structure of the human ATP synthase” online on Molecular Cell, a classic sub-journal of the world-class academic journal Cell, in collaboration with ShanghaiTech University, Guangzhou Lab, University of Queensland and others. They are the first in the world to report the high-resolution electron microscopy structure of four conformations of human ATP synthase, laying an important foundation for the understanding of its functional mechanism and related diseases as well as the development of targeted drugs.
In this study, the researchers obtained a homogeneous, stable and active human ATP synthase by extensively exploring the conditions for extraction and purification of human ATP synthase. Next, the researchers employed cryo-electron microscopy to analyze the high-resolution structure of four conformations of human ATP synthase, thereby gaining a better understanding of the structure and functions of ATP synthase. ATP synthase, similar to a rotating molecular motor, consists of a “stator” and a “rotor”, with the “rotor” containing the γεδc subunit. Driven by PMF, the c subunit rotates and drives the γεδ subunit to rotate, catalyzing the formation of ATP alongside the change in the conformation of αβ subunit. In some cases, ATP synthase can also hydrolyze ATP. At this point, the c subunit will rotate reversely, acting as an ion pump to transport protons outside the membrane.
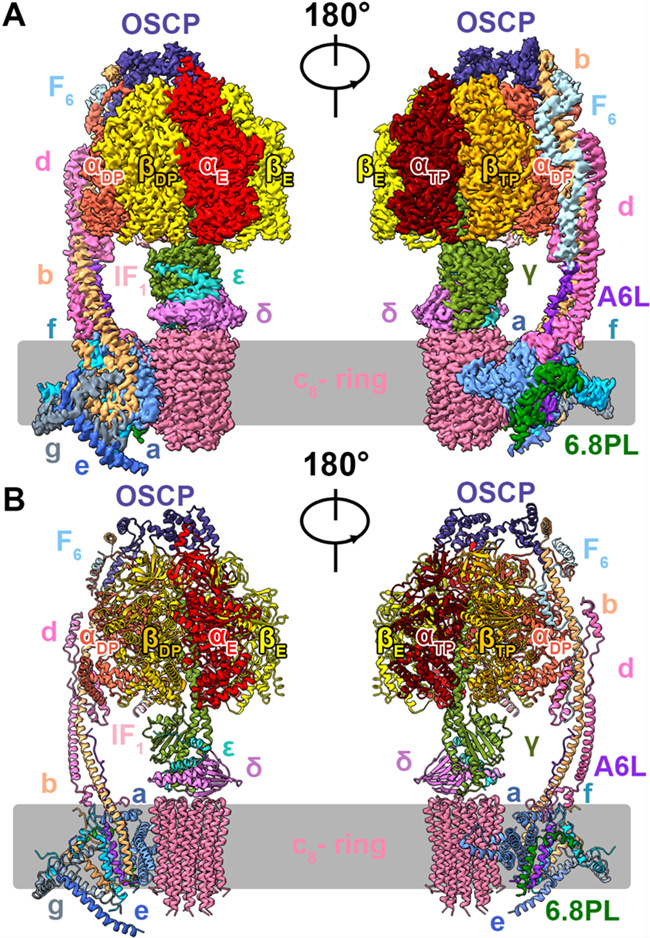
Three-dimensional structure of human ATP synthase
In this study, the structures of four rotational conformations of human ATP were analyzed. In addition to the traditional three conformations, state 3b, a sub-intermediate state, was also discovered. In the four rotation states, the β subunit exhibited significant changes in conformations. Through structural information, it was inferred that ADP release during ATP hydrolysis occurred after the β subunit was converted to open conformation, which further advanced the understanding of the rotational catalytic mechanism in the process of ATP synthase synthesis/hydrolysis of ATP.
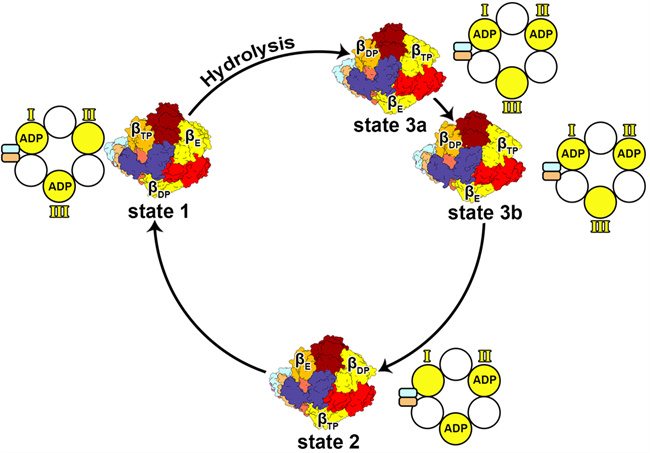
Top views of four conformations of human ATP synthase
Thanks to the high resolution, the density of water molecules in the access channel for the ATP synthase proton is clearly visible, which improves the molecular mechanism of proton access during the operation of ATP synthase. Moreover, the researchers analyzed the molecular mechanism of action related to disease-causing clinical mutations. It was found that the reported mutants mostly occurred on the contact surface between subunits, affecting the structure stability of the complex, and offering new insights for the understanding and treatment of diseases.
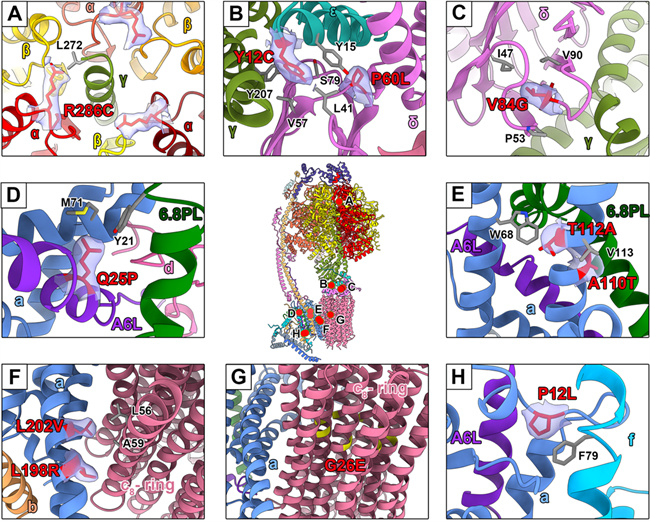
Analysis of clinical mutation sites related to human ATP synthase
It is noteworthy that ATP synthase, as the main energy source of ATP, is irreplaceable for both prokaryotes and eukaryotes, and is a popular target for drug development. Moreover, the study found that some cancers and cancer cell subsets rely on oxidative phosphorylation of mitochondria for bioenergetic and biosynthetic needs. Recent studies also confirmed that glycosylated macrolides can be used to treat leukemia through targeted inhibition of ATP synthase. Therefore, the analysis of the structure of human ATP synthase will lay the groundwork for the development of better-targeted anti-infective drugs and anti-tumor drugs with lower side effects.
Lai Yuezheng, Zhang Yuying, Zhou Shan, Xu Jinxu and Du Zhanqiang, who are doctoral students of Nankai University, are the co-first authors of this paper. Gong Hongri, professor of Nankai University College of Life Sciences; Rao Zihe, an academician; Gao Yan, associate professor of Shanghai Institute for Advanced Immunochemical Studies of ShanghaiTech University; and Liu Fengjiang, associate professor of Guangzhou Lab, are co-corresponding authors. This program was funded by the National Key Research and Development Program’s Young Scientist Program and the National Natural Science Foundation of China’s Excellent Young Scientists Fund. The imaging sub-platform of Guangzhou Lab and the Bio-Electron Microscopy Facility of ShanghaiTech University provide important technical support.
Link: https://www.cell.com/molecular-cell/fulltext/S1097-2765(23)00324-6